Healing a broken bone is no small feat and requires a highly sophisticated, coordinated series of interactions, many of which are orchestrated by the immune system. In previous posts in this osteoimmunology series, we discussed the shared aspects of bone and the immune system: a common niche, an origin story from the same progenitor cells within the bone marrow, and shared mediators (2). Now, we explore more specifically how these interactions influence bone healing.
Primary Healing: This is an uncommon method of bone repair and involves aligning the broken edges of the bone under compression and re-establishing the physical continuity at the fracture site, while limiting motion. The bone can then heal via direct remodeling of lamellar bone and Haversian canals by osteoblasts and osteoclasts.
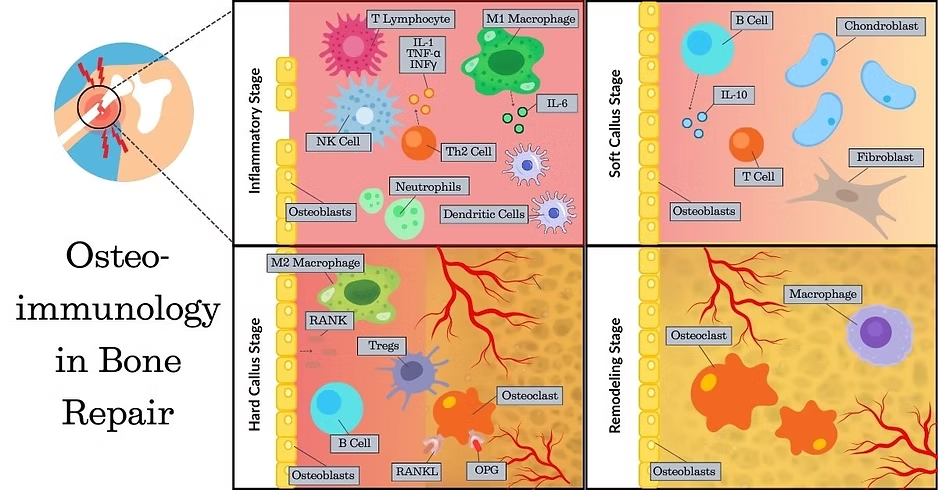
Secondary Healing: This is the most common method of bone repair and encompasses four main stages:
Acute inflammatory response leading to hematoma formation. This is important to bring macrophages, monocytes, and lymphocytes to the area to clean up fracture debris and secrete cytokines to attract mesenchymal stem cells (MSC) and other progenitor cells for bone repair.
Soft callus formation – a fibrocartilaginous network formed from MSC and their differentiated progeny: fibroblasts, osteoblasts, and chondroblasts, stabilizes the fracture.
Bony callus formation – the cartilaginous callus undergoes endochondral ossification to form the hard callus due to activity of chondroblasts, osteoblasts, and osteoclasts. New blood vessels migrate into the repair site, a process known as angiogenesis. This stage may take a month or more.
Remodeling – the bony callus is remodeled via osteoclasts and osteoblasts to restore the fractured bone to a similar strength, shape, and size as before the injury. The remodeling process may take months to years (3).
Macrophages have a key role in bone repair or fusion. They are recruited by chemokines like CXCL12 from nearby sites like the periosteum and local bone marrow, or other reserves such as the circulation or distant bone marrow.
Natural killer (NK) cells, one of the first-responder immune cells reaching the site of injury, release CXCL7, a chemokine that stimulates a three-fold increase in MSC migration to the injured site.
Pro-inflammatory macrophages (M1) produce cytokines such as tumor necrosis factor alpha (TNF-α), transforming growth factor beta (TGF-β), and interleukins (IL-1β and IL-6). These cytokines stimulate a downstream cascade leading to increased chemotaxis and proliferation of MSC and fibroblasts, osteoblast differentiation, angiogenesis, and extracellular matrix collagen synthesis.
Anti-inflammatory macrophages (M2) release cytokines like IL-4 and IL-10, as well as VEGF and matrix metalloproteinases. Together, these factors promote angiogenesis and tissue remodeling.
T cells secrete RANKL to facilitate recruitment, differentiation, and activation of osteoclasts to remove the fibrin thrombus as the hematoma is converted to the soft callus. They also promote collagen deposition by osteoblasts.
Regulatory T cells (Tregs) upregulate the differentiation of MSC to osteoblasts and downregulate osteoclast differentiation and function. Tregs modulate macrophage polarization.
B cells secrete osteoprotegerin (OPG), which modulates the effect of RANKL and osteoclast activity. For more information, see our previous post on osteoimmune crosstalk.
The initial pro-inflammatory reaction serves as an initiator of the healing cascade and plays a significant role in the bone healing process. The subsequent anti-inflammatory signaling is crucial for proceeding to the next phase, revascularization and remodeling of the fractured region. Vascularization is important for blood flow, and bone tissue grows where blood flows.
Your immune system does more than just defend, it has a starring role on the osteoimmunology team, working to maintain bone health and rebuild and restore fractured bones when necessary. Our team at Molecular Matrix, Inc. is rewriting the osteoimmunology playbook, pioneering advanced repair straties to transform bone healing and reduce disability worldwide. These innovations aren’t just reshaping science – they may be shaping your future, too!
Wu, A.-Min., Bisignano, C., James, S.L., et al. Global, regional, and national burden of bone fractures in 204 counties and territories, 1990-2019: a systematic analysis from the Global Burden of Disease Study. The Lancet Healthy Longevity. 2021. Volume 2, Issue 9, e580 – e592. https://www.thelancet.com/journals/lanhl/article/PIIS2666-7568(21)00172-0/fulltext
Cai, L., Lv, Y., Yan, Q., & Guo, W. Cytokines: The links between bone and the immune system. Injury. 2024. 55(2), 111203. https://doi.org/10.1016/j.injury.2023.111203
El-Jawhari, J. J., Jones, E., & Giannoudis, P. V. The roles of immune cells in bone healing; what we know, do not know and future perspectives. Injury. 2016. 47(11), 2399–2406. https://doi.org/10.1016/j.injury.2016.10.008
Bucher, C. H., Schlundt, C., Wulsten, D., Sass, F. A., Wendler, S., Ellinghaus, A., Thiele, T., Seemann, R., Willie, B. M., Volk, H.-D., Duda, G. N., & Schmidt-Bleek, K. Experience in the Adaptive Immunity Impacts Bone Homeostasis, Remodeling, and Healing. 2019. Frontiers in Immunology, 10, 797. https://doi.org/10.3389/fimmu.2019.00797
Lo Sicco, C., & Tasso, R. Harnessing Endogenous Cellular Mechanisms for Bone Repair. Frontiers in Bioengineering and Biotechnology 2017, 5, 52. https://doi.org/10.3389/fbioe.2017.00052
Maruyama, M.; Rhee, C.; Utsunomiya, T.; Zhang, N.; Ueno, M.; Yao, Z.; Goodman, S. B. Modulation of the Inflammatory Response and Bone Healing. Front. Endocrinol. 2020, 11, 386. https://doi.org/10.3389/fendo.2020.00386.
Schlundt, C.; Schell, H.; Goodman, S. B.; Vunjak-Novakovic, G.; Duda, G. N.; Schmidt-Bleek, K. Immune Modulation as a Therapeutic Strategy in Bone Regeneration. J. Exp. Orthop. 2015, 2 (1), 1-10. https://doi.org/10.1186/s40634-014-0017-6.
Yang, N.; Liu, Y. The Role of the Immune Microenvironment in Bone Regeneration. Int. J. Med. Sci. 2021, 18 (16), 3697–3707. https://doi.org/10.7150/ijms.61080.
https://www.molecularmatrix.com/post/osteoimmunology-bridging-bone-and-immune-system-interactions
https://www.molecularmatrix.com/post/from-infection-to-inflammation-how-immunity-shapes-bone-health
© 2025 Molecular Matrix, Inc. All rights reserved.