Innate immunity: the first line of defense, acting immediately after injury or antigen exposure. It’s nonspecific and antigen-independent, providing rapid protection.
Adaptive immunity: The second line of defense, marked by the generation of antibodies (B-cells) and cytokines (T-cells). It’s antigen-specific and requires time to mount a response, but it creates immunologic memory for faster reactions in the future.
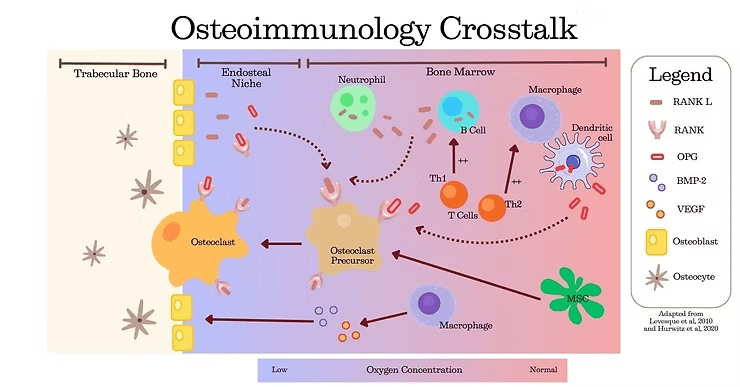
The RANK/RANKL/OPG complex is a critical pathway linking immune and skeletal systems. It regulates bone remodeling and repair by balancing bone resorption and formation (2):
RANK (Receptor Activator of Nuclear Factor Kappa-B): a receptor on the osteoclast precursors that promotes their differentiation and activation for bone resorption
RANKL (RANK Ligand): Produced by osteoblasts, osteocytes, and stromal cells, it binds to RANK to stimulate osteoclast activity, ensuring damaged bone is cleared during repair.
OPG (Osteoprotegerin): A decoy receptor secreted by osteoblasts and stromal cells, it binds to RANKL to inhibit osteoclast activation, limiting bone resorption.
Neutrophils: Express high levels of RANKL, promoting osteoclastic bone resorption.
Monocytes: Differentiate into macrophages which:
Drive the inflammation occurring at the injury site during the initial phase of healing.
Resolve inflammation and coordinate tissue regeneration in later stages.
Present antigens to T cells and secrete inflammatory mediators to recruit other immune cells.
Dendritic Cells: Trigger the cytotoxic T cell response during the initial inflammation phase (4,5).
T-helper (Th) Cells:
Th1 Cells: Activate macrophages and eliminate intracellular pathogens through cytokines like IL-12 and TNF-α.
Th2 Cells: Facilitate the removal of extracellular microorganisms and stimulate B cells, granulocytes, and mast cells.
B lymphocytes: Present antigens to activate T cells and secrete cytokines like RANKL, contributing to osteoclastogenesis.
Dendritic cells: Produce OPG to limit osteoclast activity during the later stages of bone repair (5).
Macrophages: Transition from pro-inflammatory to anti-inflammatory states, secreting growth factors like TGF-β and VEGF, which promote tissue regeneration and angiogenesis. They also release BMP-2 to stimulate osteoblast activity.
Understanding the crosstalk between bones and the immune system is key to maintaining bone health, augmenting bone repair, and developing novel treatments for inflammatory bone diseases. Curious to learn more? Explore our blog series to see how science and innovation are shaping the future of bone health.
Marshall, J. S., Warrington, R., Watson, W., & Kim, H. L. (2018). An introduction to immunology and immunopathology. Allergy, Asthma & Clinical Immunology, 14(S2), 49. https://doi.org/10.1186/s13223-018-0278-1
Udagawa, N., Koide, M., Nakamura, M. et al. Osteoclast differentiation by RANKL and OPG signaling pathways. J Bone Miner Metab 39, 19–26 (2021). https://doi.org/10.1007/s00774-020-01162-6
Bergin, S. M., Crutcher, C. L., Keeler, C., Rocos, B., Haglund, M. M., Michael Guo, H., Gottfried, O. N., Richardson, W. J., & Than, K. D. (2023). Osteoimmunology: Interactions With the Immune System in Spinal Fusion. International Journal of Spine Surgery, 17(S3), S9–S17. https://doi.org/10.14444/8556
Fischer, V., & Haffner-Luntzer, M. (2022). Interaction between bone and immune cells: Implications for postmenopausal osteoporosis. Seminars in Cell & Developmental Biology, 123, 14–21. https://doi.org/10.1016/j.semcdb.2021.05.014
Molitoris, K. H., Huang, M., & Baht, G. S. (2024). Osteoimmunology of Fracture Healing. Current Osteoporosis Reports. https://doi.org/10.1007/s11914-024-00869-z
Lévesque JP, Helwani FM, Winkler IG. The endosteal ‘osteoblastic’ niche and its role in hematopoietic stem cell homing and mobilization. Leukemia. 2010 Dec;24(12):1979-92. doi: 10.1038/leu.2010.214. Epub 2010 Sep 23. PMID: 20861913.
Hurwitz SN, Jung SK, Kurre P. Hematopoietic stem and progenitor cell signaling in the niche. Leukemia. 2020 Dec;34(12):3136-3148. doi: 10.1038/s41375-020-01062-8. Epub 2020 Oct 19. PMID: 33077865.
© 2025 Molecular Matrix, Inc. All rights reserved.