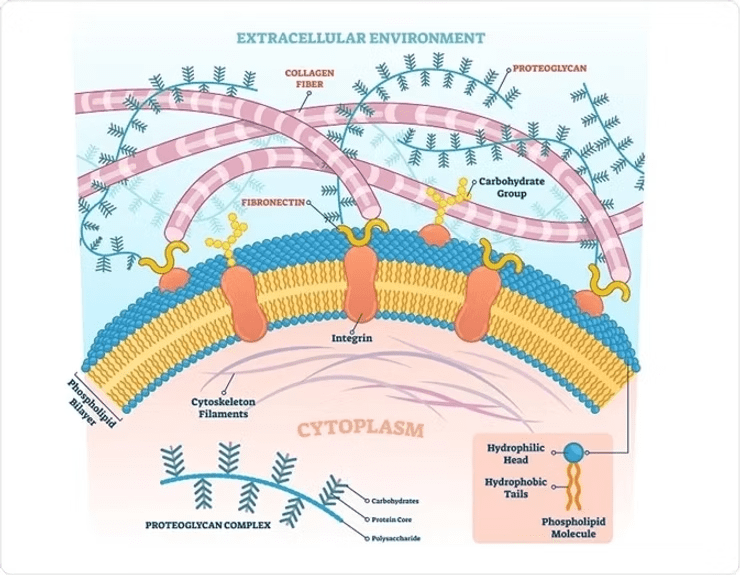
Proteoglycans (PGs) are massive supramolecular complexes, often exceeding 200 megadaltons (MDa) in size, and play a critical role in the bone extracellular matrix. These molecules regulate cross-talk between cells and the extracellular matrix through various signaling pathways. PGs consist of a protein core covalently bonded to one or more glycosaminoglycan (GAG) side chains. They mediate the binding and release of numerous signaling molecules, such as growth factors and morphogens, thereby modulating their activity and bioavailability.
Bone Morphogenetic Proteins (BMPs) are potent inducers of osteoblast differentiation from mesenchymal stem cells and play a pivotal role in bone formation. BMP-2, for instance, stimulates the expression of key transcription factors like Osterix and Runx-2 which are essential for osteoblast differentiation. It also promotes the expression of alkaline phosphatase, collagen type I, and osteocalcin. During endochondral ossification, BMPs and their receptors, expressed by chondrocytes and the surrounding perichondrium, are essential for mesenchymal stem cell differentiation into chondrocytes and subsequent stages of bone development. (Koosha & Eames, 2022; Kim & Lee, 2023; Koosha et al., 2024)
PGs may either enhance or inhibit BMP signaling. HSPGs, for example, deactivate the BMP signaling pathway by acting as co-receptors, thereby blocking BMP interaction with its receptors. Conversely, betaglycan and perlecan can activate BMP signaling by increasing the binding of BMP to its receptors. Other PGs, such syndecan-3, biglycan, and decorin, also play significant roles in modulating BMP activity and subsequent osteogenic activity. The size, charge, and chemical composition of the chondroitin and dermatan sulfate side groups are thought to contribute to the ability of these PGs to sequester BMP2, thereby regulating its signaling actions. (Miguez et al., 2011; Koosha & Eames, 2022; Koosha et al., 2024)
Designing Biomaterials for Bone Regeneration: Biomaterials for bone repair or regeneration can be modified to include PGs or PG-mimetic molecules, controlling the release of growth factors like BMP-2 or PDGF. (Martino et al., 2015)
Modifications in PG and Ligand Interaction: Modification of PG structures or ligand amino acid sequences can alter binding affinities, influence cell signaling and bioactivity, and provide specialized tissue engineering options. (Koosha & Eames, 2022)
Therapeutics: Future treatments for skeletal defects or bone repair may focus on growth factors with consideration of the PG role in modulating therapeutic activity.
Advanced Biomaterial Designs for Bone Regeneration: Bone graft substitutes, such as Osteo-PTM BGS, may be designed to mimic PGs, providing novel options to control both the proteoglycan matrix and growth factor sequestration at the repair site. For more information, refer to Koleva et al., 2019.
Abramsson A, Kurup S, Busse M, Yamada S, Lindblom P, Schallmeiner E, Stenzel D, Sauvaget D, Ledin J, Ringval, M, Landegren U, Kjellén L, Bondjers G, Li J, Lindahl U, Spillmann D, Betsholtz C, Gerhardt H. (2007). Defective N-sulfation of heparan sulfate proteoglycans limits PDGF-BB binding and pericyte recruitment in vascular development. Genes & Development, 21(3), 316–331. https://doi.org/10.1101/gad.398207
Caplan A, Correa D. (2011). PDGF in bone formation and regeneration: New insights into a novel mechanism involving MSCs. Journal of Orthopaedic Research, 29(12), 1795–1803. https://doi.org/10.1002/jor.21462
Kim KD, Lee CC. (2023). Osteogenic Cells and Microenvironment of Early Bone Development and Clinical Implication. In J. Jin Wang, G. Wang, X. Lv, Z. Sun, & K. Sunil Mahapure (Eds.), Frontiers in Spinal Neurosurgery. IntechOpen. https://doi.org/10.5772/intechopen.1002037
Koleva PM, Keefer JH, Ayala AM, Lorenzo I, Han CE, Pham K, Ralston SE, Kim KD, Lee CC. Hyper-Crosslinked Carbohydrate Polymer for Repair of Critical-Sized Bone Defects. Biores Open Access. 2019 Jul 1;8(1):111-120. doi: 10.1089/biores.2019.0021. PMID: 31346493; PMCID: PMC6657362.
Koosha E, Eames BF. (2022). Two Modulators of Skeletal Development: BMPs and Proteoglycans. Journal of Developmental Biology, 10(2), 15. https://doi.org/10.3390/jdb
Koosha E, Brenna CTA, Ashique AM, Jain N, Ovens K, Koike T, Kitagawa H, Eames BF. (2024). Proteoglycan inhibition of canonical BMP-dependent cartilage maturation
delays endochondral ossification. Development (Cambridge, England), 151(2), dev201716. https://doi.org/10.1242/dev.201716
Martino MM, Briquez PS, Maruyama K, Hubbell JA. (2015). Extracellular matrix-inspired growth factor delivery systems for bone regeneration. Advanced Drug Delivery Reviews, 94, 41–52. https://doi.org/10.1016/j.addr.2015.04.007
Miguez PA, Terajima M, Nagaoka H, Mochida Y, Yamauch, M. (2011). Role of glycosaminoglycans of biglycan in BMP-2 signaling. Biochemical and Biophysical Research Communications, 405(2), 262–266. https://doi.org/10.1016/j.bbrc.2011.01.022
Shah P, Keppler L, Rutkowski J. (2014). A Review of Platelet Derived Growth Factor Playing Pivotal Role in Bone Regeneration. Journal of Oral Implantology, 40(3), 330–340. https://doi.org/10.1563/AAID-JOI-D-11-00173
Zafiropoulos A, Fthenou E, Chatzinikolaou G, Tzanakakis GN. (2008). Glycosaminoglycans and PDGF Signaling in Mesenchymal Cells. Connective Tissue Research, 49(3–4), 153–156. https://doi.org/10.1080/03008200802148702
© 2025 Molecular Matrix, Inc. All rights reserved.