The smallest bone in the human body is roughly the size of a grain of rice! The stapes, a stirrup-shaped bone inside your middle ear, may be tiny, but it plays a huge role in hearing by transmitting sound vibrations to the inner ear. Bones are often unnoticed, yet bone fractures are one of the most common reasons for emergency room visits. In today’s post, we explore the science behind fracture repair and the natural healing mechanisms of bones.
Bones are often taken for granted. They are strong, yet flexible, and provide structure, protection, and movement. Yet we rarely think about our bone health. Each of our 206 bones (approximate) has it’s own unique shape, size and purpose. Long bones, like the humerus, femur and phalanges, handle weight-bearing and mobility. Flat bones protect internal organs and include the skull, ribs, and scapula. Short bones provide support and flexibility in joints such as the wrist and ankle and include examples like the carpal and tarsal bones. Bones with complex shapes that don’t fit other categories are known as irregular bones, and include bones of the face, spine and pelvis. Sesamoid bones are small, round bones embedded in tendons. One example is the patella (kneecap) which provides protection and mechanical advantages. Despite their strength, bones are not invincible.
Bone fractures are the most common traumatic injury and a leading cause of emergency room visits, affecting individuals of all ages and activity levels (1). Young athletes may suffer collarbone (clavicle) fractures from contact sports injuries while older adults are more prone to hip fractures due to falls and age-related bone loss. Wrists, ankles, and forearms are common fracture sites, often resulting from falls, accidents, and repetitive stress injuries (2).
Fortunately, bones have an intrinsic repair mechanism and are capable of gradually repairing and restoring strength to the fracture site. However, depending on the severity and type of break, surgical intervention may be required to realign, stabilize, and support healing. From the initial impact to full recovery, understanding how bones heal can help in managing fractures effectively (3-5). In this post, we explore the science behind fracture repair, the body’s natural healing mechanisms, and modern advancements in treatment.
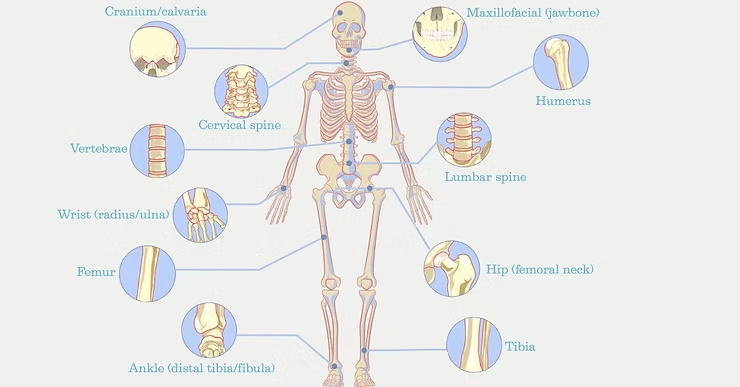
Understanding Bone Fractures
Simple vs Comminuted Fractures: A simple fracture results from bending or twisting forces, breaking the bone into two segments. A comminuted fracture, however, occurs from high-impact trauma, shattering the bone into multiple fragments. While simple fractures generally heal through spontaneous repair, comminuted fractures may lead to long-term deformities if not therapeutically addressed (6).
Stress Fractures: Caused by repetitive loading and microdamage over time, stress fractures occur due to prolonged exposure to low-magnitude cyclic forces. These fractures heal through normal bone remodeling mechanisms (6).
Open vs Closed Fractures: Open fractures involve a break in the skin, usually causing greater damage to the adjoining soft tissues and periosteum, while having a higher risk of infection and non-union (6). Closed fractures do not penetrate the skin which reduces risk of infection, but still involves pain and swelling at the injury site.
Stages of bone fracture healing (3-6,8,9):
I. Acute inflammatory response (~ 5 days)
Hematoma formation occurs and coagulates to form a temporary scaffold, which further serves as a template for callus formation.
Immune cells, inflammatory markers, and osteogenic growth factors are released.
II. Fibrocartilaginous network or soft callus formation (days 5 – 10 post fracture)
This stage involves the recruitment and differentiation of mesenchymal stem cells (MSCs) into osteoblasts, fibroblasts, and chondroblasts.
Deposition of a soft callus or collagen-rich fibrocartilaginous network.
III. Bony callus formation ( ~ 4 weeks post injury)
The soft or cartilaginous callus undergoes endochondral ossification (resorption of the soft callus and deposition of woven bone).
IV. Bone remodeling (~ months to years)
The bony callus undergoes remodeling via osteoblasts and osteoclasts forming compact bone (in the center) and lamellar bone (in the periphery).
Bone remodeling helps in attaining the rigidity and biomechanical stability of the normal bone.
When the fracture gap, or defect, is too large to heal spontaneously, this is termed a critical-size defect. Generally, these defects measure 2- 2.5 cm in size in humans. A nonunion fracture is a type of fracture that fails to heal properly, often due to secondary conditions like diabetes or osteoporosis. In these cases, additional bone reconstruction procedures are employed to bridge the gap and support repair.
Autograft: The patient’s own bone, usually from the hip (iliac crest), is transplanted to the defect site. This is the current gold standard but comes with potential complications at the harvest site and possible limitations on the amount of bone that can be safely harvested.
Allograft: Bone tissue from a donor is used to provide a framework for growth of bone tissue. Despite stringent processing and sterilization regulations, there is some risk of disease transmission and/or infection.
Bone graft substitutes: This type of graft uses biomaterials of varying composition, porosity, and surface properties to serve as a scaffold for bone repair. Bone graft substitutes can be optimized for a range of applications and may also serve as carriers for bioactive molecules or cells to aid in healing.
Understanding the different biological phases of fracture repair allows clinicians to develop personalized treatment approaches for efficient recovery and to minimize the duration of immobilization required. Research into biomaterials and signal-driven bone regeneration continues to drive innovations in bone graft substitutes and treatments for non-union fractures. At Molecular Matrix, Inc., our expertise in bone regeneration supports the development of cutting-edge biomaterials for enhanced bone healing. Look for future posts on this topic and learn more about our innovative solutions at www.molecularmatrix.com.
Ho-Shui-Ling, A., Bolander, J., Rustom, L. E., Johnson, A. W., Luyten, F. P., & Picart, C. (2018). Bone regeneration strategies: Engineered scaffolds, bioactive molecules and stem cells current stage and future perspectives. Biomaterials, 180, 143–162. https://doi.org/10.1016/j.biomaterials.2018.07.017
GBD 2019 Fracture Collaborators. Global, regional, and national burden of bone fractures in 204 countries and territories, 1990-2019: a systematic analysis from the Global Burden of Disease Study 2019. Lancet Healthy Longev. 2021;2(9):e580-e592. doi:10.1016/S2666-7568(21)00172-0
D.C. Boyd, The anatomical basis for fracture repair: Recognition of the healing continuum and its forensic applications to investigations of pediatric and elderly abuse, in: C.C. Boyd, D.C. Boyd (Eds.), Forensic Anthropol., 1st ed., Wiley, 2018: pp. 149–200. https://doi.org/10.1002/9781119226529.ch9.
K. D. Kim, C. C. Lee, Osteogenic Cells and Microenvironment of Early Bone Development and Clinical Implication, in: J. Jin Wang, G. Wang, X. Lv, Z. Sun, K. Sunil Mahapure (Eds.), Front. Spinal Neurosurg., IntechOpen, 2023. https://doi.org/10.5772/intechopen.1002037.
J.M. Kanczler, J.A. Wells, D.M.R. Gibbs, K.M. Marshall, D.K.O. Tang, R.O.C. Oreffo, Bone tissue engineering and bone regeneration, in: Princ. Tissue Eng., Elsevier, 2020: pp. 917–935. https://doi.org/10.1016/B978-0-12-818422-6.00052-6.
A. Bigham‐Sadegh, A. Oryan, Basic concepts regarding fracture healing and the current options and future directions in managing bone fractures, Int. Wound J. 12 (2015) 238–247. https://doi.org/10.1111/iwj.12231.
C.E. Baker, S.N. Moore-Lotridge, A.A. Hysong, S.L. Posey, J.P. Robinette, D.M. Blum, M.A. Benvenuti, H.A. Cole, S. Egawa, A. Okawa, M. Saito, J.R. McCarthy, J.S. Nyman, M. Yuasa, J.G. Schoenecker, Bone Fracture Acute Phase Response—A Unifying Theory of Fracture Repair: Clinical and Scientific Implications, Clin. Rev. Bone Miner. Metab. 16 (2018) 142–158. https://doi.org/10.1007/s12018-018-9256-x.
H. ElHawary, A. Baradaran, J. Abi-Rafeh, J. Vorstenbosch, L. Xu, J.I. Efanov, Bone Healing and Inflammation: Principles of Fracture and Repair, Semin. Plast. Surg. 35 (2021) 198–203. https://doi.org/10.1055/s-0041-1732334.
I. Pountos, P.V. Giannoudis, Fracture Healing: Back to Basics and Latest Advances, in: P.V. Giannoudis (Ed.), Fract. Reduct. Fixat. Tech., Springer International Publishing, Cham, 2018: pp. 3–17. https://doi.org/10.1007/978-3-319-68628-8_1.
© 2025 Molecular Matrix, Inc. All rights reserved.