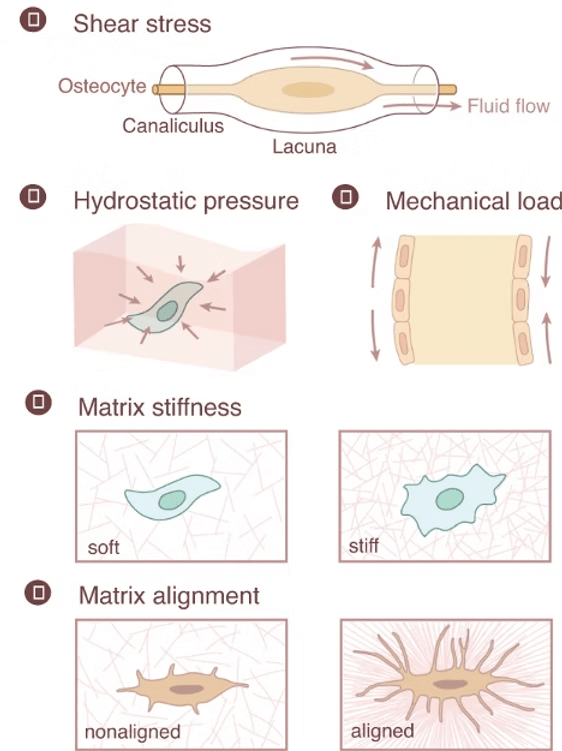
Osteocytes: These mature bone cells are nestled in the mineralized matrix, connected by channels that sense fluid shifts during movement. Changes in fluid shear stress prompt osteocytes to signal other cells to either build bone when under load stress or increase bone resorption when unstressed. (Qin et al., 2020; Xu et al., 2021)
Mesenchymal Stem Cells (MSCs): Fluid shear stress encourages MSCs to differentiate into osteoblasts and suppresses the formation of osteoclasts. (Datta et al., 2006; Henstock et al., 2024)
Osteoclasts: These bone-resorbing cells have ion channels sensitive to fluid stress that regulate their activity, morphology, and gene expression. (Bratengeier et al., 2020)
Osteoblasts: These bone-building cells respond to physical load by producing proteins and growth factors that promote bone matrix formation and stabilization at injury sites. (Knapik et al., 2014)
MSC differentiation can be switched from adipogenesis to osteogenesis by changing ECM stiffness.
Osteoblast maturation increases on firm ECM while soft ECM appears to favor osteoclast maturation.
Focal adhesions: These large macromolecular protein complexes can contain over 100 different proteins and have roles in cell migration. Integrins are a crucial transmembrane component of focal adhesions. These heterodimeric receptors functionally link the ECM to the cytoskeleton, providing a means to transfer mechanical stimuli from the extracellular environment to intracellular signaling or cytoskeletal proteins.
Primary Cilium: Mechanical signals such as fluid shear stress are detected by cilium and transmitted via the hedgehog signaling pathway. In bone, this signals osteoprogenitors to differentiate into bone-forming osteoblasts at certain frequencies.
G protein-coupled receptors: These receptors, located on the cell membrane, sense mechanical stimuli and transduce the signal via the Rho-Rock and PLC-IP3 signaling pathways. (Kalinkovich & Livshits, 2018)
Stretch-Activated Ion channels: Ion flow, especially calcium influx, modulates downstream signaling. (Martino et al., 2018)
Cytoskeleton: Within the cell is a dynamic network of interlinking protein filaments and tubules known as the cytoskeleton. In addition to providing for cell shape, organization of organelles and cell movement, the cytoskeleton provides a pathway for mechanical forces to be transferred from the plasma membrane to internal cellular structures. (Maniotis et al., 1997)
Controlled physical load during rehabilitation can stimulate bone healing by strengthening the initial callus and promoting complete remodeling. Advanced biomaterials and implants are being developed to mimic mechanical cues, helping guide cell behavior and optimize healing. (Hart et al., 2017)
Mechanical signals are a crucial component of the body’s repair toolkit. Understanding and leveraging these forces could enable us to better harness the healing potential of orthopedic tissues. At Molecular Matrix, Inc., our bone graft substitute Osteo-P® BGS models the natural bone extracellular matrix, providing substrate and mechanical cues for repair with the patient’s own cells. Through these cellular conversations, we’re developing new possibilities in regenerative medicine that are both innovative and aligned with the body’s intrinsic healing abilities.
Bratengeier, C., Liszka, A., Hoffman, J., Bakker, A. D., & Fahlgren, A. (2020). High shear stress amplitude in combination with prolonged stimulus duration determine induction of osteoclast formation by hematopoietic progenitor cells. FASEB journal : official publication of the Federation of American Societies for Experimental Biology, 34(3), 3755–3772. https://doi.org/10.1096/fj.201901458R
Datta, N., Pham, Q. P., Sharma, U., Sikavitsas, V. I., Jansen, J. A., & Mikos, A. G. (2006). In vitro generated extracellular matrix and fluid shear stress synergistically enhance 3D osteoblastic differentiation. Proceedings of the National Academy of Sciences of the United States of America, 103(8), 2488–2493. https://doi.org/10.1073/pnas.0505661103
Hart NH, Nimphius S, Rantalainen T, Ireland A, Siafarikas A, Newton RU. Mechanical basis of bone strength: influence of bone material, bone structure and muscle action. J Musculoskelet Neuronal Interact. 2017 Sep 1;17(3):114-139. PMID: 28860414; PMCID: PMC5601257.
Henstock, J. R., Price, J. C. F. A., & El Haj, A. J. (2024). Determining Which Hydrostatic Pressure Regimes Promote Osteogenesis in Human Mesenchymal Stem Cells. Tissue engineering and regenerative medicine, 10.1007/s13770-024-00666-w. Advance online publication. https://doi.org/10.1007/s13770-024-00666-w
Kalinkovich, A., & Livshits, G. (2021). Biased and allosteric modulation of bone cell-expressing G protein-coupled receptors as a novel approach to osteoporosis therapy. Pharmacological research, 171, 105794. https://doi.org/10.1016/j.phrs.2021.105794
Knapik, D. M., Perera, P., Nam, J., Blazek, A. D., Rath, B., Leblebicioglu, B., Das, H., Wu, L. C., Hewett, T. E., Agarwal, S. K., Jr, Robling, A. G., Flanigan, D. C., Lee, B. S., & Agarwal, S. (2014). Mechanosignaling in bone health, trauma and inflammation. Antioxidants & redox signaling, 20(6), 970–985. https://doi.org/10.1089/ars.2013.5467
Maniotis, A. J., Chen, C. S., & Ingber, D. E. (1997). Demonstration of mechanical connections between integrins, cytoskeletal filaments, and nucleoplasm that stabilize nuclear structure. Proceedings of the National Academy of Sciences of the United States of America, 94(3), 849. https://doi.org/10.1073/pnas.94.3.849
Martino, F., Perestrelo, A. R., Vinarský, V., Pagliari, S., & Forte, G. (2018). Cellular Mechanotransduction: From Tension to Function. Frontiers in Physiology, 9, 378185. https://doi.org/10.3389/fphys.2018.00824
Qin, L., Liu, W., Cao, H. et al. Molecular mechanosensors in osteocytes. Bone Res 8, 23 (2020). https://doi.org/10.1038/s41413-020-0099-y
Robling, A. G., Castillo, A. B., & Turner, C. H. (2006). Biomechanical and molecular regulation of bone remodeling. Annual review of biomedical engineering, 8, 455–498. https://doi.org/10.1146/annurev.bioeng.8.061505.095721
Xu X, Liu S, Liu H, Ru K, Jia Y, Wu Z, Liang S, Khan Z, Chen Z, Qian A, Hu L. Piezo Channels: Awesome Mechanosensitive Structures in Cellular Mechanotransduction and Their Role in Bone. Int J Mol Sci. 2021 Jun 16;22(12):6429. doi: 10.3390/ijms22126429. PMID: 34208464; PMCID: PMC8234635.
© 2025 Molecular Matrix, Inc. All rights reserved.