A twofold increased risk of developing osteoporosis.
Two-to-threefold higher risk of hip fractures.
Two-to-sixfold higher risk of vertebral fractures.
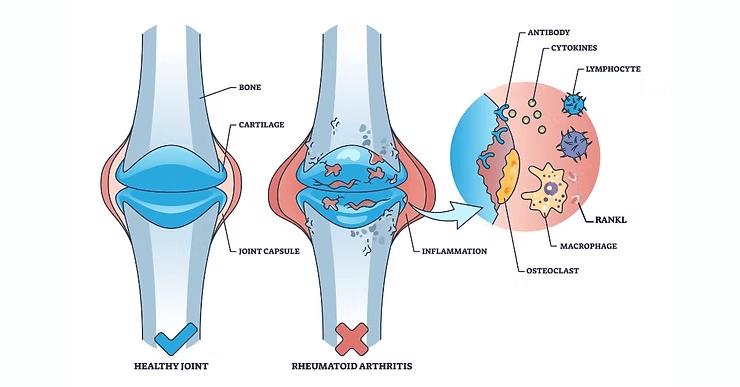
Synovial inflammation in RA leads to destruction of bone and cartilage in joints (7).
Periarticular bone loss (osteopenia) occurs early, while systemic bone loss involves both the appendicular and axial skeleton. The accelerated loss of cortical and cancellous bone makes RA patients more prone to osteoporotic fractures (8).
Inflammatory cytokines such as TNF-α, IL-1β, IL-6, and IL-17 contribute to osteoclast activation and simultaneously suppress osteogenesis (8).
T-helper 17 cells (Th17 cells, named for synthesis of IL-17) are abundant in the inflamed synovium and amplify the secretion of inflammatory cytokines. This, in turn, promotes local inflammation and osteoclast differentiation. Th17 also triggers RANKL expression, further promoting bone resorption (7,8).
Synovial fibroblasts, stimulated by IL-17, upregulate RANKL expression, fueling osteoclast activity. Circulating monocytes migrate to inflamed joints, where pro-inflammatory cytokines facilitate their transformation into osteoclasts (2,7-9).
B lymphocytes exacerbate bone loss by interacting with osteoclast Fcγ receptors and promoting osteoclastogenesis. Meanwhile, Tumor Necrosis Factor (TNF) suppresses osteoblast-driven bone formation via inhibition of Wnt signaling through dickkopf-related protein 1 (DKK1) and sclerostin, further restraining bone regeneration (7).
Autoantibodies such as rheumatoid factor (RF) and anti-citrullinated protein antibodies (ACPA) initiate early structural bone damage, even in the preclinical phase of RA. Inflammation accelerates this process by amplifying osteoclast formation and bone resorption, highlighting that RA progression begins long before clinical symptoms appear (3,5,8).
Joint synovium autoantibodies form immune complexes, activating complement pathways that intensify inflammation and tissue destruction. Chronic inflammation further induces angiogenesis, recruiting more inflammatory cells, and systemic cytokines may trigger extra-articular effects such as rheumatoid nodules and muscle protein degradation (6).
Amarasekara, D. S., Yu, J., & Rho, J. (2015). Bone Loss Triggered by the Cytokine Network in Inflammatory Autoimmune Diseases. Journal of Immunology Research, 2015, 1–12. https://doi.org/10.1155/2015/832127
El Khassawna, T., Serra, A., Bucher, C. H., Petersen, A., Schlundt, C., Könnecke, I., Malhan, D., Wendler, S., Schell, H., Volk, H.-D., Schmidt-Bleek, K., & Duda, G. N. (2017). T Lymphocytes Influence the Mineralization Process of Bone. Frontiers in Immunology, 8, 562. https://doi.org/10.3389/fimmu.2017.00562
Ingegnoli, F., Castelli, R., & Gualtierotti, R. (2013). Rheumatoid Factors: Clinical Applications. Disease Markers, 35, 727–734. https://doi.org/10.1155/2013/726598
Kamen, D. L., & Alele, J. D. (2010). Skeletal manifestations of systemic autoimmune diseases. Current Opinion in Endocrinology, Diabetes & Obesity, 17(6), 540–545. https://doi.org/10.1097/MED.0b013e328340533d
Liu, J., Gao, J., Wu, Z., Mi, L., Li, N., Wang, Y., Peng, X., Xu, K., Wu, F., & Zhang, L. (2022). Anti-citrullinated Protein Antibody Generation, Pathogenesis, Clinical Application, and Prospects. Frontiers in Medicine, 8, 802934. https://doi.org/10.3389/fmed.2021.802934
Lončar, S. R., Halcrow, S. E., & Swales, D. (2023). Osteoimmunology: The effect of autoimmunity on fracture healing and skeletal analysis. Forensic Science International: Synergy, 6, 100326. https://doi.org/10.1016/j.fsisyn.2023.100326
Okamoto, K. (2024). Crosstalk between bone and the immune system. Journal of Bone and Mineral Metabolism, 42(4), 470–480. https://doi.org/10.1007/s00774-024-01539-x
Schett, G. (2017). Autoimmunity as a trigger for structural bone damage in rheumatoid arthritis. Modern Rheumatology, 27(2), 193–197. https://doi.org/10.1080/14397595.2016.1265907
Umur, E., Bulut, S. B., Yiğit, P., Bayrak, E., Arkan, Y., Arslan, F., Baysoy, E., Kaleli-Can, G., & Ayan, B. (2024). Exploring the Role of Hormones and Cytokines in Osteoporosis Development. Biomedicines, 12(8), 1830. https://doi.org/10.3390/biomedicines12081830
© 2025 Molecular Matrix, Inc. All rights reserved.