The Science Behind Bone Graft Substitutes: How They Work
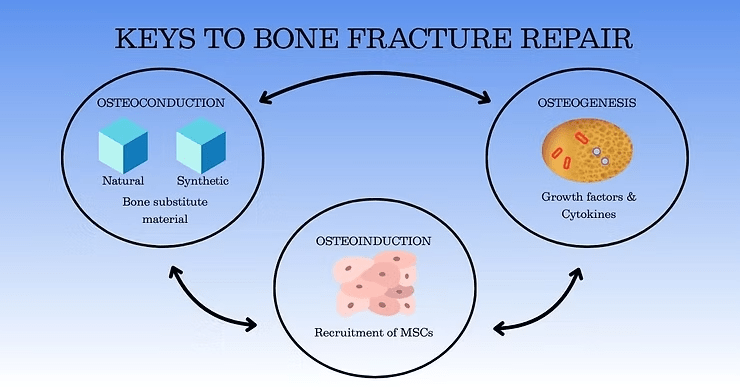
Osteogenesis: Direct Bone Formation
The osteogenic properties of a bone graft determine its ability to generate new bone tissue from the graft itself. Osteogenic grafts contain the osteoprogenitor cells, growth factors and matrix that are required to form new bone.
Fresh autografts and bone marrow aspirates retain living osteoblasts or MSC, enabling direct new bone formation.
Allografts and synthetic materials lack osteogenic cells but can support bone formation when paired with osteoinductive signaling molecules (1-4).
Osteoinduction: A Key Step in Activating Bone Formation
Osteoinduction refers to the ability of a graft to stimulate osteoprogenitor cells such as mesenchymal stem cells (MSC) to differentiate into bone-forming osteoblasts and chondroblasts, leading to new bone formation. This process is regulated by factors that orchestrate cell differentiation, proliferation, growth, and matrix deposition, including:
Bone Morphogenetic Proteins (BMP)
Fibroblast Growth Factors (FGF)
Platelet-derived Growth Factor (PDGF)
Vascular Endothelial Growth Factor (VEGF)
Others
Osteoconduction: Providing a Scaffold for Growth
Osteoconduction refers to the ability of the graft to serve as a scaffold, allowing new bone cells to adhere, proliferate, and integrate with the patient’s existing bone. As discussed in our last post, BGS with osteoconductive properties include:
Porous ceramic-based substitutes like hydroxyapatite and β-TCP that provide structural support while permitting cell infiltration.
Bioactive materials like synthetic polymers that encourage capillary formation and cellular migration.
Next-generation synthetic carbohydrate polymers, like Osteo-P® BGS, that support cell and vascular infiltration, and sequester the patient’s own osteoinductive factors for bone formation.
Inflammatory Response – Recruitment of immune cells to the graft site.
Secretion of Growth Factors – MSC and macrophages drive tissue remodeling.
Cellular Infiltration and Scaffold Utilization – The patient’s own MSC, osteoprogenitors, and vascular progenitors migrate into the graft via the porous scaffold. In the case of autografts, live cells within the graft itself may contribute to repair.
Osteoblast Differentiation – Growth factors and cytokines induce differentiation of these cells into osteoblasts and chondroblasts, which begin the process of bone repair.
New Bone Synthesis and Revascularization – Osteoblasts synthesize new bone while circulation is re-established by vasculogenesis. Vascularization is a key to long-term integration as tissue grows where blood flows.
Matrix remodeling and graft resorption – Over time, mature bone replaces the graft material as the bone’s natural cellular remodeling processes take over (1,5).
High osteogenic potential due to the presence of osteoblasts and osteocytes.
Faster incorporation due to high porosity and vascularization (6-12 months).
Autografts undergo direct resorption while allografts may form a fibrous capsule which slows mineralization.
Cortical bone is denser than cancellous bone, is more frequently used as a structural or load-bearing bone graft, and has a slower integration process.
Integration occurs through a process known as creeping substitution, a slow resorption of the graft with deposition of new bone.
Creeping substitution is driven by osteoclasts and begins at the graft-host junction.
The process occurs over many years, leading to initial mechanical strength loss before full integration.
At Molecular Matrix, Inc., we apply tissue engineering principles to develop advanced bone graft substitutes that enhance healing, integration, and patient recovery. Learn more about our cutting-edge solutions at www.molecularmatrix.com
Georgeanu, V. Al., Gingu, O., Antoniac, I. V., & Manolea, H. O. (2023). Current Options and Future Perspectives on Bone Graft and Biomaterials Substitutes for Bone Repair, from Clinical Needs to Advanced Biomaterials Research. Applied Sciences, 13(14), 8471. https://doi.org/10.3390/app13148471
Sohn, H.-S., & Oh, J.-K. (2019). Review of bone graft and bone substitutes with an emphasis on fracture surgeries. Biomaterials Research, 23(1), 9. https://doi.org/10.1186/s40824-019-0157-y
Gillman, C. E., & Jayasuriya, A. C. (2021). FDA-approved bone grafts and bone graft substitute devices in bone regeneration. Materials Science and Engineering: C, 130, 112466. https://doi.org/10.1016/j.msec.2021.112466
Inglis, J. E., Goodwin, A. M., Divi, S. N., & Hsu, W. K. (2023). Advances in Synthetic Grafts in Spinal Fusion Surgery. International Journal of Spine Surgery, 17(S3), S18–S27. https://doi.org/10.14444/8557
Ranjan Dahiya, U., Mishra, S., & Bano, S. (2019). Application of Bone Substitutes and Its Future Prospective in Regenerative Medicine. In M. Barbeck, O. Jung, R. Smeets, & T. Koržinskas (Eds.), Biomaterial-supported Tissue Reconstruction or Regeneration. IntechOpen. https://doi.org/10.5772/intechopen.85092
© 2025 Molecular Matrix, Inc. All rights reserved.