Part 3: Electrical Signals
The electrical potential of bone is a intriguing aspect of its physiology. Let’s first explore terms used to describe electrical potentials involving cells and tissues, including bone:
Dielectric: The ability to store and dissipate electrical energy. The cell membrane, composed of a lipid bilayer, typically acts as an electrical insulator but can be polarized in the presence of an electrical force, contributing to its dielectric behavior.
Piezoelectric: Electric charge produced in response to mechanical stress, primarily attributed to the crystal structure of collagen molecules in the bone.
Pyroelectric: The ability of bone to generate an electric charge in response to temperature changes.
Ferroelectric: Spontaneous electric polarization which is reversible with the application of an external electric field.
These potentials work together with physiological ion movements to produce interactive crosstalk between the organic and inorganic components of bone and between bone cells and their extracellular matrix, playing roles in bone metabolism, homeostasis, and regeneration.
Electroactive and Electrosensitive Constituents of Bone Cells
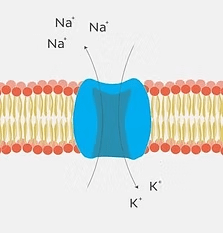
Cellular Transmembrane Potential: Differences in sodium and potassium ion concentrations create a transmembrane electrical field in bone cells, altering cell metabolism and signaling pathways controlling bone tissue homeostasis and remodeling.
Voltage-Sensitive Ion Channels: Sodium, potassium, calcium, and chloride ion channels are present in bone cells and respond to electrical stimuli with ion flux that affects bone maintenance, deposition, and resorption.
Intracellular Signaling Pathways: Electrical stimuli may activate or inhibit intracellular signaling pathways which, in turn, influence downstream gene and protein expression.
Extracellular Matrix Proteins: Structural characteristics of the bone’s extracellular matrix enhance its electrical properties, contributing to its dielectric, piezoelectric, pyroelectric, and ferroelectric properties.
Hydroxyapatite: Influences bone’s bioelectrical properties by restricting the hydrogen bonding of collagen fibers with water molecules, influencing collagen fiber orientation and mechanical response to compressive forces. Hydration status plays a role here too, as water-saturated collagen fibers align more symmetrically, short-circuiting the electrical potential.
Osteocytes, the most abundant cells in bone tissue, play a crucial role in maintaining bone health. They are embedded within the mineralized bone matrix and have long, dendritic-like cell processes that extend through the canaliculi to form gap junctions with adjacent osteocytes and osteoblasts. The result is a functional syncytium of interconnected cells throughout bone tissue that acts to transmit electrical and other signals.
One of the key mechanisms by which bone cells communicate electrically is through mechanotransduction. This process involves the conversion of mechanical stimuli, such as strain or pressure, into electrical signals. When bone tissue is subjected to mechanical stress, it generates electrical potentials known as streaming potentials. These potentials are detected by osteocytes, then relayed to other bone cells via the molecules and mechanisms discussed above.
Electrical communication plays a crucial role in bone metabolism, homeostasis, and regeneration. The dielectric, piezoelectric, pyroelectric, and ferroelectric properties of bone enable response to various stimuli and coordination of cellular activities. The electroactive constituents of bone cells, including transmembrane potentials and various channels and proteins, transmit and/or enhance this communication. As research continues to uncover the intricacies of electrical signaling in bone cells, new therapeutic approaches for orthopedic tissue repair and bone-related disorders may emerge.
Look for our next post where we will discuss the application of electrical signaling in bone healing therapeutics!